ADALA Trial: DOACs Match DAPT in Thrombosis Prevention but Cut Bleeding Risk
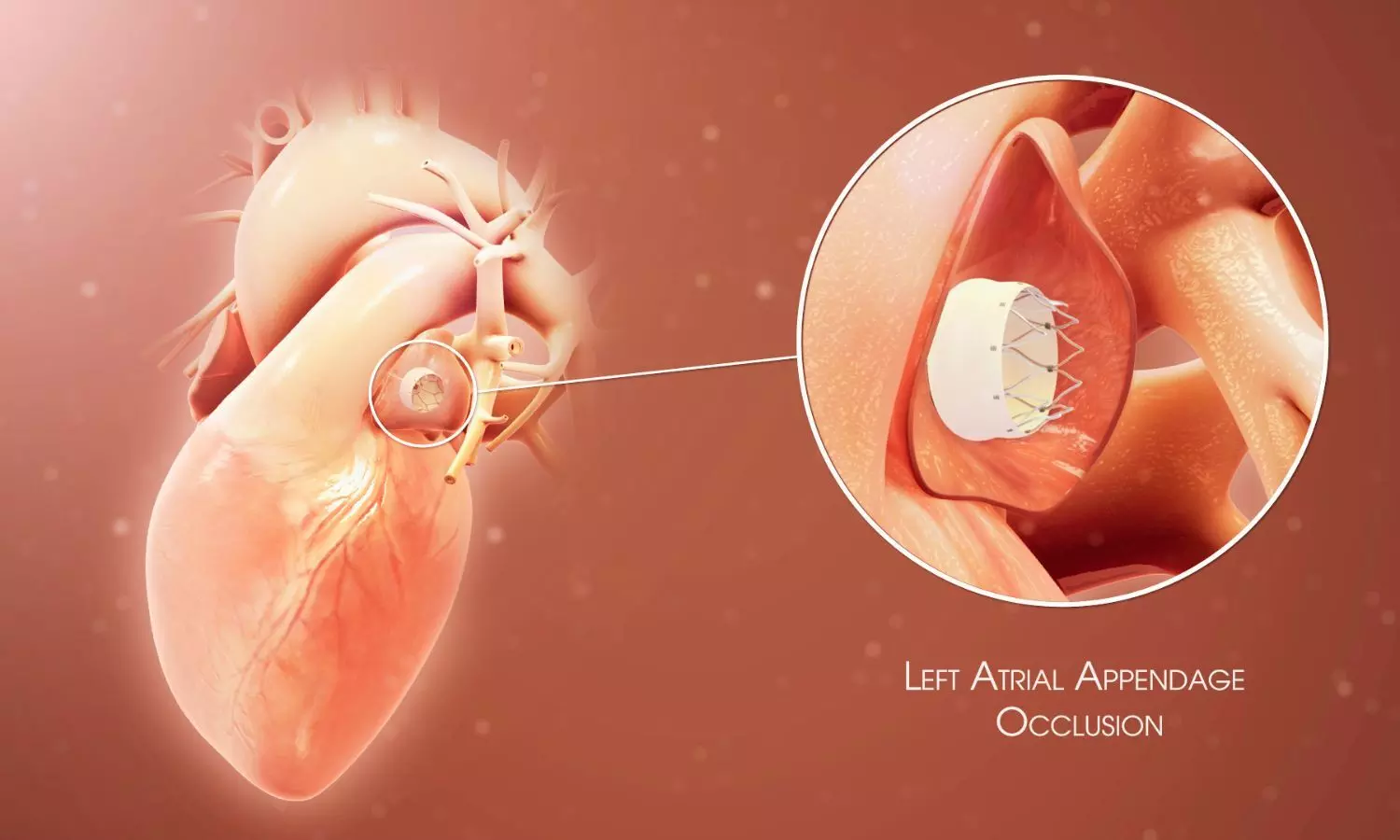
USA: The final results of the ANDES (Antithrombotic Therapy for the Prevention of Device-Related Thrombosis After Left Atrial Appendage Occlusion) trial, presented at the Transcatheter Cardiovascular Therapeutics (TCT) 2025 conference, have offered important insights into post-procedural therapy after Left Atrial Appendage Occlusion (LAAO).
The findings suggest that Direct Oral Anticoagulants (DOACs) are as effective as dual antiplatelet therapy (DAPT) in preventing device-related thrombosis (DRT) while significantly reducing the risk of bleeding.
LAAO is often performed in patients with non-valvular atrial fibrillation who are at high risk of stroke but unsuitable for long-term anticoagulation. Following device implantation, there remains a potential for thrombosis formation on the device surface. Traditionally, physicians have prescribed aspirin and clopidogrel for one to three months after the procedure. However, this standard dual antiplatelet therapy carries a bleeding risk, particularly in older adults and patients with multiple comorbidities. The ANDES trial aimed to determine whether a DOAC-based approach could provide equivalent protection against thrombosis with better safety.
The trial was a multicenter, randomized, controlled study enrolling patients who had undergone successful LAAO. Participants were randomly assigned to receive either a DOAC or a DAPT regimen immediately after the procedure. The primary efficacy measure was the incidence of device-related thrombosis at 60 days, while major and clinically relevant non-major bleeding events were the main safety endpoints. Most patients enrolled were elderly, had high CHA₂DS₂-VASc scores, and were considered at high risk for both thromboembolic and bleeding events.
Findings
from the trial were as follows:
- After
60 days, there was no significant difference in device-related thrombosis rates between the DOAC and DAPT groups. - Overall
event rates were low and aligned with previous registry data. - Patients
on DOACs experienced fewer bleeding complications, including
gastrointestinal and access-site bleeding. - The
DOAC group showed a lower rate of net adverse clinical events. - Short-term
safety outcomes were better in patients treated with DOACs.
According to the study’s principal investigator, while DOACs did not reduce thrombosis rates compared to DAPT, they clearly offered a superior safety profile. The results indicate that a short course of DOAC therapy could serve as a safer alternative to DAPT following LAAO, especially for patients at high risk of bleeding.
The findings also highlight that factors beyond systemic anticoagulation—such as device design, incomplete endothelialization, or peri-device leaks—may play a greater role in thrombosis formation. Despite similar efficacy, the safety advantage of DOACs makes them a practical option for post-LAAO care.
Researchers emphasized that larger and longer-duration studies are needed to confirm whether the short-term safety benefits of DOACs translate into improved long-term outcomes, including stroke prevention and late device-related thrombosis. Until then, the ANDES trial contributes valuable evidence supporting personalized post-LAAO antithrombotic strategies that balance efficacy with safety in patients with atrial fibrillation.
Reference:
Facebook Comments

