15 Drugs Including Pomol-650, Glimiz-2 Banned in Karnataka, Check Full List

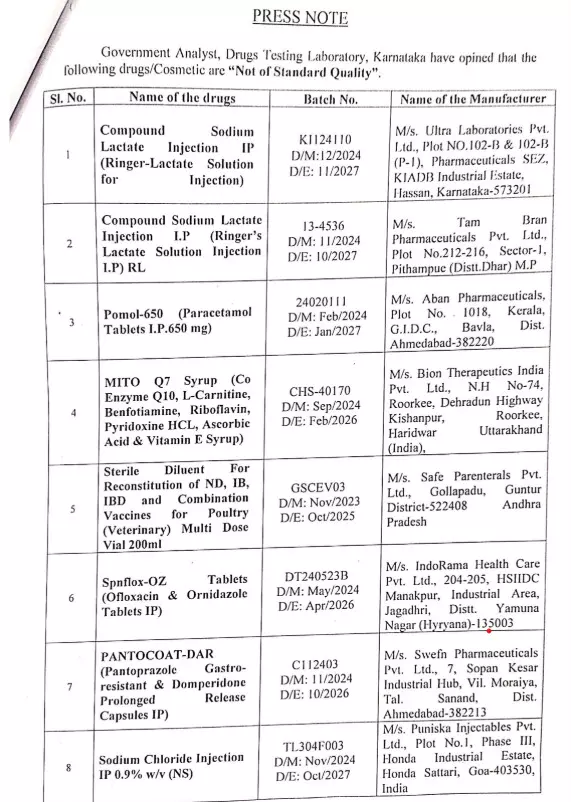
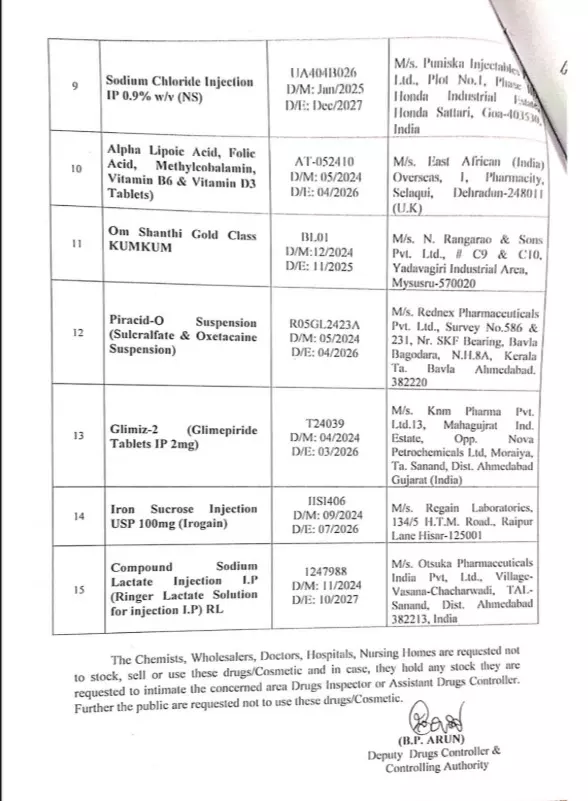
Bengaluru: In a move to protect public health, the Karnataka Government’s Drug Testing Laboratory has declared 15 drugs manufactured by 14 companies as “Not of Standard Quality”.
Authorities have issued an urgent directive to chemists, wholesalers, doctors, hospitals, and nursing homes, instructing them not to stock, sell, or use the listed drugs. Those already in possession of these products are required to intimate the concerned area Drugs Inspector or Assistant Drugs Controller. The public has also been strongly advised not to use these medicines.
Some of the notable substandard drugs include Compound Sodium Lactate Injection IP (Ringer-Lactate solution for injection) manufactured by Ultra Laboratories, Otsuka Pharmaceuticals India and Tam Bran Pharmaceuticals, Pomol-650 (Paracetamol Tablets IP 650 mg) by Aban Pharmaceuticals, and MITO Q7 Syrup by Bion Therapeutics India.
The list also includes Glimiz-2 (Glimepiride tablets IP 2mg) manufactured by Knm Pharma, Iron Sucrose Injection USP 100 mg (Irogain) manufactured by Regain Labs.