Both CPP-ACPF and sodium fluoride have protective effect on Enamel, suggests study
Researchers have found in new research that both casein phosphopeptide-amorphous calcium phosphate fluoride (CPP-ACPF) and sodium fluoride (NaF) showed a protective effect on tooth enamel against erosion caused by an orange juice-based beverage.
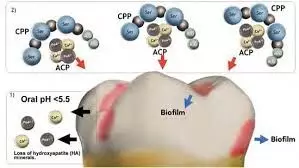
With the increasing prevalence of dental erosion, this study explores the protective role of traditional fluoride-based products and newer formulations on eroded enamel. A study was done to assess the protective effectiveness of casein phosphopeptide-amorphous calcium phosphate fluoride (CPP-ACPF) and sodium fluoride (NaF) on human enamel against erosion using surface microhardness (SMH) and Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM) analyses. Ten extracted human third molars were sectioned to obtain 40 enamel sections and randomly assigned into four groups (n = 10) and treated as follows: G1 (Sound Enamel), G2 (Erosive Challenge), G3 (CPP-ACPF + Erosive Challenge), and G4 (NaF + Erosive Challenge). All samples were subjected to Vicker’s SMH analysis, while changes in surface morphology and elemental composition were validated in few representative samples using FTIR and SEM, respectively. Results:
The mean SMH1 values for the experimental groups G3 and G4 were significantly higher (426.58VHN and 455.83VHN) when compared to G1 (P = 0.000) and G2 (P = 0.000). In SEM analysis, G2 showed eroded honeycomb appearance compared to the smooth homogenous surface of G1, while both G3 and G4 showed deposition of some precipitates. FTIR analysis revealed that in G3 and G4, a characteristic peak of phosphate vibrations between 528 and 823 cm-1 and carbonate bands at 845-932 cm-1 was observed. Both CPP-ACPF and NaF demonstrated a protective effect on enamel against erosive challenge by an orange juice-based beverage.
Reference:
Assadi, Mohammed Raihan; Devadiga, Darshana; Ingle, Aditya; Jain, Nainy; Devadiga, Dheeraj1. Efficacy of Sodium Fluoride and Fluoridated Calcium Phosphate in Mitigating Dental Erosion on Human Enamel: An In vitro Analysis. Indian Journal of Dental Research 35(3):p 309-314, Jul–Sep 2024. | DOI: 10.4103/ijdr.ijdr_80_24
Keywords:
Assadi, Mohammed Raihan; Devadiga, Darshana; Ingle, Aditya; Jain, Nainy; Devadiga, Dheeraj, Both, CPP-ACPF, sodium, fluoride, protective, effect, Enamel, suggests study
Powered by WPeMatico