Ventilation in hospitals could cause viruses to spread further, study suggests
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

Kolkata: The Calcutta High Court on Thursday directed registrar of the West Bengal Medical Council (WBMC), to voluntarily resign from his position by 5 p.m. on January 31. The court noted that he had been holding the post for over five years, despite retiring in 2019, and without the necessary approval from the state government..
The Calcutta High Court on Thursday ordered the West Bengal Medical Council’s Registrar Manas Chakraborty to immediately vacate the post.
A division bench of Chief Justice T.S. Sivagnanam and Justice Hiranmay Bhattacharya has also cautioned Chakraborty that unless he himself resigns by 5 p.m. on Friday (January 31), he will be removed from the position by the court.
According to an IANS report, the court gave this direction following the observation that he had been occupying that chair for five years even after his retirement. It also questioned how he could continue in that chair for such a long time without approval from the state government.
Although Chakraborty’s term as the West Bengal Medical Council Registrar ended in November 2019, he continued occupying the chair even after that. As per the provision of the Bengal Medical Act, 1914, the prior approval of the state government is necessary for the extension of anybody’s term in that chair – a practice which was not followed in the case of Chakraborty.
Also Read:West Bengal Medical Council Registrar removed amid allegations of Illegal Appointment
The division bench gave this direction on Thursday acting on a petition filed at the court challenging the extended tenure of Chakraborty as well as accusing rampant irregularities in the functioning of the council during his term, reports IANS.
While giving the direction for the immediate removal of Chakraborty from his chair, the division bench observed that the status of the state medical council cannot be above the state government.
Since the members of the council are in a noble profession, they should act accordingly, the division bench further observed.
Chakraborty had recently faced criticism for issuing a show-cause notice to Asfaqulla Naiya, a prominent figure in the junior doctors’ movement over the R.G. Kar rape and murder case. Naiya was accused of conducting private practice as an ENT specialist without possessing the necessary degree.
A police investigation was also started against Naiya. However, a single-judge bench of the Calcutta High Court earlier this month had provided him with an interim shield against any coercive police action against him, including arrest.
Also Read:Controversy Erupts Among WB Medical Council Staff Over New Registrar Appointment
Powered by WPeMatico

Hyderabad: In yet another digital arrest scam, the Hyderabad Cyber Crime Police have arrested two individuals from Gujarat for conning a 54-year-old female doctor out of Rs. 3 crore by posing as government officials.
The two suspects, both aged 50, were involved in 17 similar fraud cases across India, with additional cases still under investigation. One of the accused works as a home loan consultant, while the other is a property dealer, and both are based in Gujarat.
The scam began when the doctor, based in Gudimalkapur, Hyderabad, received a phone call from an individual claiming to be an officer from the Telecom Department. The caller informed her that her mobile number would be blocked within two hours because it was allegedly linked to a money laundering case in Delhi. Despite the doctor’s denial of the allegations, the caller escalated the situation by transferring the call to Skype, where she was connected to a person who identified himself as an IPS officer from Delhi.
This individual presented a fake court order, supposedly issued by Chief Justice of India D.Y. Chandrachud, and instructed the doctor to prove her innocence by depositing large sums of money into designated bank accounts.
Also Read:Cops save doctor from falling victim to Digital Arrest Scam
Speaking about the case with The Hindu, The police said, “The doctor was threatened with the safety of her family if she did not comply. She was assured that the money would be refunded once her innocence was proven, with the added warning that the matter was a ‘National Secret’ and should not be disclosed to anyone.”
Following the threats, the doctor fearing for her family’s safety, redeemed her fixed deposits and transferred a total of Rs. 3 crore into the bank accounts provided by the Faudstars.
The case came to light when the doctor reported the incident to the Hyderabad Cyber Crime Police, leading to an investigation and the eventual arrest of the accused individuals.
The police investigation revealed that the accused used various tactics to deceive their victims. They impersonated representatives from government bodies such as the Telecom Department, TRAI, CBI, and even the Cyber Crime Police, making calls via WhatsApp, Skype, and other internet-based services. The scammers threatened victims with digital arrest, accusing them of serious crimes like money laundering or terrorism.
Also Read: Sassoon Hospital doctor conned by cyber fraudsters, loses Rs 25 lakh
Powered by WPeMatico

An Instagram reel claims that Blood Pressure can get normal by onion juice. The claim by the user is Misleading.
In an Instagram reel it is claimed that Blood Pressure can get normal by onion juice. The reel by the user fried_moni is titled “High BP ke liye home remedy” In the reel the user says, “In case of BP, if you want to normalize BP, take an onion, grate it, and extract its juice. Alright? Every morning, what you need to do is drink one spoon of onion juice. After that, don’t eat or drink anything for half an hour, and take one spoon in the evening as well. This will help a lot with BP.”
The reel has received 760,000 views, 10,841 likes, 36 comments, and 7,289 shares, and is available here.
The claim by the user is Misleading. Onions may assist in managing high blood pressure; however, no scientific evidence or medical consensus supports the claim that onion juice can normalize blood pressure.
According to WHO, High blood pressure or Hypertension is when the pressure in blood vessels is high (140/90 mmHg or higher). It is common but can be serious if not treated.
The risk factors of hypertension are genetics, overweight or obese, physical inactivity, age, high-salt diet and consumption of alcohol.
Modifiable risk factors include unhealthy diets such as excessive salt consumption, a diet high in saturated fat and trans fats, low intake of fruits and vegetables, physical inactivity, consumption of tobacco and alcohol, and being overweight or obese. In addition, there are environmental risk factors for hypertension and associated diseases, where air pollution is the most significant.
Non-modifiable risk factors include a family history of hypertension, age over 65 years and co-existing diseases such as diabetes or kidney disease.

Dr. Pradeep Hasija, Consultant, Cardiology, Jaslok Hospital & Research Centre explained, “Hypertension is most important modifiable risk factor for cardiovascular disease and affects nearly 30% of world’s adult population. In large majority of adult patients the cause of hypertension is obesity, family history, salt intake and certain medications. Hypertension related to lifestyle factors are likely to remit with nonpharmacological measures like weight reduction, regular exercise, salt reduction, cessation of smoking and avoidance of tea, coffee or stimulant drugs. Another category of hypertension called secondary hypertension that is due to hormonal abnormalities (cortisol, thyroid or catecholamins), sleep disorder (obstructive sleep apnoea), obstruction in aorta (Coarctation) and certain kidney disease (renal artery stenosis, fibromuscular dysplasia , parenchymal disease) are potentially reversible with treatment of underlying disease. A DASH diet (dietary advice to stop hypertension) is based on eating more vegetables, fruits, and whole grains and eating less salt and saturated fat and is recommended for blood pressure reduction. The adoption of yoga, meditation and stress relieving exercises have also important role to play in reversal of hypertension.”
High blood pressure can be controlled with lifestyle modifications, diet and certain medications as prescribed by a registered medical practitioner, but there is no cure for hypertension.
Onions add abundant flavor to a wide variety of food, yet are low in calories. With only 45 calories per serving, onions are naturally fat and cholesterol-free. They are a source of dietary fiber, vitamin C, vitamin B6, potassium, and other key nutrients including folate, calcium and iron. Onions contain a variety of other naturally occurring chemicals known as organosulfur compounds linked to lowering blood pressure and cholesterol levels.
Among some of their best-known benefits, onions contain the flavonoid quercetin which acts as an anti-inflammatory in the body, inhibits low-density lipoprotein oxidation (an important reaction in atherosclerosis and coronary heart disease), protects and regenerates vitamin E (a powerful antioxidant).
Onions contain certain bioactive compounds that may have beneficial effects in the management of high blood pressure but there is no scientific evidence or medical consensus to support the claim that drinking onion juice can normalize blood pressure. It is important to emphasize that high blood pressure cannot be completely cured but can only be effectively managed.
Onions may contribute to the effective management of high blood pressure. According to a review by Amin Galavi et. al., onion has been shown to positively influence metabolic health parameters, including high blood pressure, diabetes, and obesity.
Quercetin, a natural compound found in onions, has shown promising effects in helping in the management of hypertension. A randomized trial led by Verena Brüll et. al. revealed that quercetin supplementation from onion skin extract significantly lowered ambulatory blood pressure in individuals with hypertension, emphasizing its potential role in cardiovascular protection.
While onions contain compounds like quercetin, which may have a mild impact in the management of blood pressure, there is no robust scientific evidence or medical consensus to support the idea that onion juice alone can normalize blood pressure.

The Medical Dialogues Fact Check Team spoke with Dr Prem Aggarwal, MD (Medicine), DNB (Cardiology, Director, Sanjeevan Hospital, Daryaganj, Delhi and he said, “While onions contain antioxidants like quercetin, which may help in managing hypertension, drinking onion juice alone is unlikely to normalize blood pressure. Blood pressure management requires a holistic approach, including a balanced diet, regular exercise, stress reduction, and medication if prescribed. Relying solely on home remedies like onion juice may delay essential medical care. Always consult a doctor for effective and safe hypertension management.”
Dr Pradeep Hasija, Consultant, Cardiology, Jaslok Hospital & Research Centre further said, “Onion juice have received great attention as a dietary supplement to benefit blood pressure, however it lacks scientific evidence. It does make a great addition to a blanched diet being
low in calories, fat, and sodium, and rich in antioxidant flavonoids (quercetin) and fibers. In a recent observational study published in Lancet – it was shown that 42% elderly achieved remission and were off medication for over 2 years. They were nonsmoker to start with and achieving normal body mass index during follow-up, and quitting alcohol drinking during follow-up, among others were associated with remission of hypertension. The decrease in BP was also attributed to an increased level of frailty in older adults. Hypertension therefore, can be controlled by medication and in some can remit, however lifestyle modification has an important role to play in achieving and maintaining remission.”
The claim that blood pressure can be normalized by onion juice is unsupported by any scientific evidence or medical consensus. While onions have certain health benefits, there is no conclusive research proving that onion juice alone can effectively manage or normalize blood pressure. It is crucial to note that high blood pressure is not curable but can be controlled successfully.
Hence, the claim by the user is Misleading.
Powered by WPeMatico
Enobosarm demonstrated a statistically significant benefit in preserving lean mass in patients with overweight or obesity undergoing treatment with semaglutide.
Topline results from the Phase 2b QUALITY trial indicate that enobosarm, a novel oral selective androgen receptor modulator (SARM), led to a statistically significant reduction in lean mass loss in patients receiving semaglutide (WEGOVY), a GLP-1 receptor agonist, for weight reduction.
Announced by Veru Pharmaceuticals on January 27, 2025, the trial evaluated enobosarm 3 mg and 6 mg versus placebo in adults aged ≥60 years. The study met its primary endpoint, with additional positive effects on key secondary endpoints, including changes in total fat mass, body composition, and physical function.
The Phase 2b QUALITY clinical study was a multicenter, double-blind, placebo-controlled, randomized, dose-finding study to evaluate the safety and efficacy of enobosarm 3mg, and enobosarm 6mg, compared to placebo in 168 older patients, greater than 60 years of age, who are overweight or have obesity and who are receiving WEGOVY (semaglutide), a GLP-1 receptor agonist (RA), for weight reduction. The primary endpoint was the change in total lean body mass from baseline to 16 weeks, and key secondary endpoints were the change from baseline to 16 weeks in total fat mass, total body weight, and physical function as measured by a stair climb test.
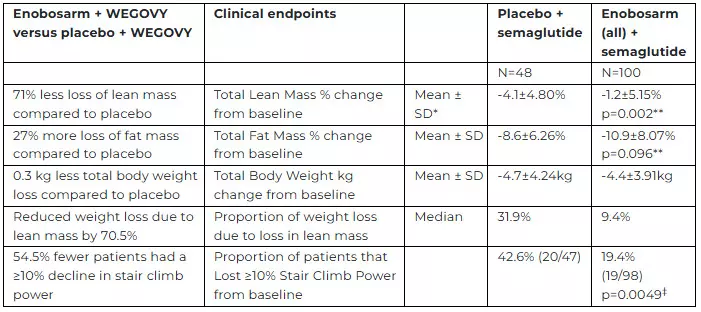
*Standard Deviation, **ANCOVA model, least square means analysis using gender and BMI at baseline as covariates, ‡ Fisher’s exact test
• The Phase 2b QUALITY study is the first human study to report the effects of a muscle preservation agent on body composition in older patients who have obesity or are overweight and receiving a GLP-1 receptor agonist.
• In the topline efficacy analysis, the Phase 2b QUALITY clinical study met its primary endpoint with a statistically significant benefit in preservation of total lean body mass in all patients receiving enobosarm + semaglutide versus placebo + semaglutide at 16 weeks.
• Secondary endpoints showed:
⋅ Enobosarm + semaglutide treatment resulted in a greater reduction in total fat mass compared to placebo + semaglutide at 16 weeks.
⋅ There appears to be minor differences in total body weight changes between the enobosarm + semaglutide group and placebo + semaglutide group at 16 weeks. Therefore, enobosarm + semaglutide improved changes in body composition and resulted in more selective and greater loss of fat mass than in subjects receiving placebo + semaglutide.
⋅ The proportion of subjects that lost ≥10% stair climb power was statistically significant and clinically meaningfully reduced in the enobosarm + semaglutide groups compared to placebo + semaglutide group. Therefore, enobosarm reduced the proportion of patients that lost clinically significant physical function versus subjects receiving semaglutide alone.
The Company plans to present the full clinical efficacy and safety data set for the Phase 2b QUALITY clinical study in future scientific conferences and publications after the Phase 2b extension portion of study is completed and unblinded.
Dr. Louis Aronne, an obesity expert, past president of the Obesity Society and a scientific advisor and consultant to Veru, who was not directly involved in the QUALITY study stated: “These topline clinical results are very exciting. Weight loss through any modality produces a loss of both lean and fat mass. The greater magnitude of weight loss seen with bariatric surgery and GLP-1 RA based drugs has produced an unmet medical need to preserve muscle and physical function in older patients receiving these treatments. This is the first clinical study to demonstrate prevention of both lean mass loss and decline in muscle function associated with weight loss in older individuals treated with a GLP-1 RA. This combined treatment approach could benefit patients with obesity and low amounts of muscle due to age-related muscle loss.”
“Given the promising topline results from the Phase 2b QUALITY clinical trial where enobosarm treatment preserved lean mass, increased fat loss, improved body composition changes, and prevented decline in stair climb physical function in patients receiving WEGOVY (semaglutide), we plan to meet with FDA to discuss the design of the Phase 3 clinical program,” said Mitchell Steiner, M.D., Chairman, President, and Chief Executive Officer of Veru. “The Phase 2b QUALITY study is the first human study to demonstrate that older patients who are overweight or have obesity and receiving only a WEGOVY (semaglutide) GLP-1 RA are at higher risk for accelerated frailty and functional decline. Lean mass loss in the semaglutide group that did not receive enobosarm was significant as 32% of the total weight loss at 16 weeks was made up of lean mass. Loss of lean mass also matters as 42.6% of patients on placebo + semaglutide had at least a 10% decline in stair climb power. The potential for further reduction in physical function because of ongoing loss of lean mass with chronic GLP-1 RA therapy is worrisome and must be evaluated. The expectation is that all GLP-1 RA containing drugs could cause significant loss of lean mass in older patients raising concerns for potential declines in physical function, mobility disability, functional limitations, and loss of balance with a higher risk for falls and fractures.”
“Older patients who have obesity or who are overweight and are receiving a GLP-1 RA are an ideal target population that have demonstrated in the Phase 2b QUALITY clinical study clinical benefit with enobosarm treatment to provide a greater quality weight loss as lean mass and physical function may be preserved with greater and selective loss of adiposity, that is, better body composition weight reduction may be possible. Further, the expectation is that when patients are treated longer with enobosarm, which results in greater loss of adiposity, there would also be a profoundly greater weight reduction than with semaglutide alone,” said Gary Barnette Ph.D., Chief Scientific Officer, Veru Inc.
Safety data remains blinded in the ongoing clinical study and the unblinded safety set will be available when the Phase 2b extension study is done in April 2025. However, the aggregate, blinded data, have not shown significant differences compared to different previous studies of enobosarm. The Independent Data Monitoring Committee also met in October 2024 and evaluated the unblinded safety data with a recommendation to continue the study as planned. As a reminder, enobosarm has a large safety database, which includes 27 clinical trials involving 1581 mostly older men and women, some of which included patients dosed for up to 3 years. In this large safety database, enobosarm was generally well tolerated with no increases in gastrointestinal side effects. This is important as there are already significant and frequent gastrointestinal side effects with a GLP-1 RA treatment alone.
As a reminder, the Phase 2b extension clinical trial where all patients will stop receiving a GLP-1 RA, but will continue taking placebo, enobosarm 3mg, or enobosarm 6mg for an additional 12 weeks is ongoing. The blinded Phase 2b extension clinical trial is asking a different question than the Phase 2b QUALITY clinical study which evaluated the ability of enobosarm to improve body composition changes associated with GLP-1 RA weight loss induction. The Phase 2b extension study will evaluate maintenance of weight loss, meaning whether enobosarm can maintain muscle and prevent the fat and weight gain that occurs after discontinuing a GLP-1 RA. If successful, this would provide another important obesity related indication for which enobosarm could be considered. The topline results for the separate blinded Phase 2b extension clinical study are expected in April of 2025.
As the Phase 2b QUALITY study has positive topline clinical results, we are planning to move forward to request an end of Phase 2 meeting with FDA. In the new weight reduction FDA guidance, FDA makes a regulatory path distinction between weight reduction drugs and drugs for body composition changes. FDA states that: “Sponsors seeking an efficacy claim related to changes in body composition would need to consult with FDA early in development to align on the clinical condition being treated. Trial design, including appropriate choice of population and selection of endpoints that measure how a patient feels, functions, or survives, to potentially support such a claim is beyond the scope of this weight reduction guidance.” Based on this updated FDA guidance, enobosarm is being developed as a body composition drug to selectively preserve lean body mass and physical function, and augment loss of fat in older patients who are overweight or have obesity receiving GLP-1 RA containing drug for chronic weight management. We have previously met with FDA to discuss our regulatory path forward as an improvement in body composition drug, and FDA has provided general advice on Phase 3 design.
Based on the Phase 2b QUALITY clinical trial, the proposed Phase 3 clinical trial design is currently expected to be a double-blind, placebo-controlled study in older (> 60 years of age), patients who have obesity or who are overweight and who are eligible for treatment with GLP-1 RA. The GLP-1 RA may be either WEGOVY (semaglutide) and/or Zepbound® (tirzepatide). Patients will be randomized to oral daily enobosarm or matching placebo. All subjects will start and receive GLP-1 RA during the study.
The proposed primary objective will be the effect of enobosarm on stair climb power, as measured by the proportion of subjects that lose ≥10% stair climb power from baseline. Proposed key secondary objectives will be to assess the effect of enobosarm on total lean mass, total body weight, total fat mass, bone mineral density, HOMA-IR, and hemoglobin A1c.
The duration of treatment is expected to be 52 weeks which will allow us to also capture the benefits of enobosarm improvements on body composition for greater loss of adiposity and weight reduction. Based on the responder rates of the stair climb power observed in this Phase 2b clinical study, the predicted trial sample size is expected to be approximately 470 total subjects with a 90% power and an alpha of 0.05.
The fully enrolled Phase 2b, multicenter, double-blind, placebo-controlled, randomized, dose-finding QUALITY clinical trial evaluated the safety and efficacy of enobosarm 3mg, enobosarm 6mg, or placebo as a treatment to preserve muscle and augment fat loss in 168 patients with sarcopenic obesity or overweight elderly (>60 years of age) patients receiving semaglutide (Wegovy®). The primary endpoint was total lean body mass, and the key secondary endpoints were total body fat mass and physical function as measured by stair climb test at 16 weeks. After completing the efficacy dose-finding portion of the Phase 2b QUALITY clinical trial, it is expected that participants will then continue in blinded fashion into a Phase 2b extension clinical trial where all patients will stop receiving a GLP-1 RA, but will continue taking placebo, enobosarm 3mg, or enobosarm 6mg for an additional 12 weeks. The Phase 2b extension clinical trial will evaluate whether enobosarm can maintain muscle and prevent the fat and weight gain that occurs after discontinuing a GLP-1 RA. The top-line results of the separate blinded Phase 2b extension clinical study are expected in the second calendar quarter of 2025.
The clinical condition to improve body composition by preserving muscle and enhancing the loss of adiposity. We believe the market for this condition is quite large. Based on Medicare statistics, 22% of the US population is over 60 years of age, and according to the CDC, 42% of older adults have obesity in the United States and could benefit from a weight loss medication. Up to 34 % of obese patients over the age of 60 have sarcopenic obesity, sarcopenia being age-related loss of muscle. This large subpopulation of sarcopenic obese patients is especially at risk when taking GLP-1 drugs for weight reduction as they may already have critically low amounts of muscle due to age-related muscle loss. Because of the magnitude and the speed of muscle loss while on GLP-1 RA therapy for weight loss, GLP-1 RA drugs may accelerate the development of frailty and muscle weakness in obese or overweight elderly patients.
Muscle weakness may lead to poor balance, decreased gait speed, mobility disability, functional limitations, loss of independence, and higher risk for falls and fractures. In fact, the safety section of the package insert for Wegovy has been updated based on the recently reported SELECT cardiovascular outcomes clinical trial which now highlights a 400% increase in pelvic and hip fractures that was observed in patients greater than 75 years of age receiving Wegovy compared to placebo (2.4% versus 0.6%). Fractures of the hip and pelvis typically occur because of falls which increase with decreased muscle mass.
Enobosarm (aka ostarine, MK-2866, GTx-024, and VERU-024), a novel oral daily selective androgen receptor modulator (SARM), has been previously studied in 5 clinical studies involving 968 older normal men and postmenopausal women as well as older patients who have muscle wasting because of advanced cancer. Advanced cancer causes the loss of appetite where there is significant unintentional loss or wasting of both muscle and fat mass which is similar to what is observed with in patients taking GLP-1 RA drugs. We believe the totality of the clinical data from these previous five clinical trials demonstrates that enobosarm treatment leads to dose-dependent increases in muscle mass with improvements in physical function as well as significant dose-dependent reductions in fat mass. The patient data generated from these five enobosarm clinical trials in both elderly patients and in patients with a cancer induced appetite suppression provide strong clinical rationale for enobosarm. The expectation is that enobosarm in combination with a GLP-1 RA would potentially augment the fat reduction and total weight loss while preserving muscle mass.
Enobosarm has a large safety database, which includes 27 clinical trials involving 1581 men and women, some of which included patients dosed for up to 3 years. In this large safety database, enobosarm was generally well tolerated with no increases in gastrointestinal side effects. This is important as there are already significant and frequent gastrointestinal side effects with a GLP-1 RA treatment alone.
Powered by WPeMatico

Age-related brain atrophy, the gradual loss of neurons and shrinkage of brain tissue, is a natural part of aging, which can lead to cognitive decline and other neurological issues. While so far aging cannot be prevented, recent research from an 18-month dietary intervention offers hope that lifestyle and dietary changes can slow brain aging. A new international study, led by Ben-Gurion University of the Negev as part of the DIRECT PLUS Brain MRI trial, has brought to light how blood sugar control can significantly impact brain health.
Brain age, as evaluated by MRI measurements of the hippocampus and lateral ventricles, reflects the biological aging of the brain, which can differ from a person’s chronological age. Chronological age is the number of years lived, while brain age indicates the brain’s actual health. Typically, as we age, the hippocampus shrinks and the lateral ventricles expand, serving as markers of brain aging. Some individuals have a brain age younger or older than their chronological age. A younger brain age suggests better cognitive health, while an older brain age may indicate accelerated aging and increased risk of cognitive decline.
The study, which was published recently in The American Journal of Clinical Nutrition, was conducted by an international team of brain and nutrition experts, including researchers from Ben-Gurion University, Harvard University, Leipzig University, and more. The research was primarily carried out by Ph.D. student Dafna Pachter and overseen by Prof. Iris Shai, along with several international collaborators.
A previous study published two years ago , reported that Mediterranean (MED) and green-MED diets significantly attenuated age-related brain atrophy by ∼50% within 18 months.
In the current study, the researchers aimed to understand the mechanism by which the slowing of brain atrophy occurs.
The study found that a decline in HbA1c, and key markers of long-term blood sugar levels, are associated with significant positive changes in specific brain regions commonly affected by age-related atrophy. Brain MRI results showed that lower HbA1c levels corresponded to greater deviations in the thalamus, caudate nucleus, and cerebellum – areas crucial for cognitive function, motor control, and sensory processing. The study suggests that improved blood sugar control could be one of the most important factors in slowing down age-related brain changes.
Earlier research has highlighted the benefits of the Green Mediterranean (Green-Med) diet, including better blood sugar control. The Green-Med diet is rich in polyphenols from plant-based sources like Mankai (a high-protein aquatic plant) and green tea, while being low in red and processed meats. The current study further strengthens this connection by suggesting that the Green-Med diet may not only support metabolic health but also exert protective effects on brain structure and function.
The DIRECT PLUS trial, one of the longest and largest brain MRI studies conducted to date, involved approximately 300 participants who were divided into three dietary groups. Whole-brain MRI measurements were taken before and after the 18-month trial to track changes in brain health. The researchers used Hippocampal Occupancy (HOC), as a proxy for brain age which predicts future risk of dementia. HOC typically decreases with age. Interestingly, some participants exhibited a brain age either younger or older than their chronological age.
Using NeuroQuant, an FDA-authorized fully automated tool, the research team quantified and segmented the brain MRI-derived data. The study aimed to examine whether improved glycemic control and specific dietary components could slow down brain aging. The results indicated that participants who managed to improve their blood sugar levels and achieve normal glucose status experienced a more pronounced attenuation of brain aging. Notably, those who consumed higher amounts of green tea and Mankai duckweed shakes demonstrated the most significant improvements in both blood sugar levels and brain health.
The study’s lead researcher, Prof. Iris Shai, from Ben-Gurion University, an adjunct professor at Harvard University, and an Honorary Professor at Leipzig University, explains, “Maintaining low blood sugar levels, even within the normal range, shows promise for preserving a younger brain, especially when combined with a healthy diet and regular physical activity. Specifically, polyphenols found in plant-based foods may cross the blood-brain barrier and help reduce brain inflammation, which is crucial for memory”.
Dafna Pachter, a Ph.D. student and the first author of the paper, adds, “This trial offers a safe approach to potentially slow down our brain aging—by adopting the components of a green-Mediterranean diet.”
This study is one of the first large-scale trials to directly link dietary changes, particularly those associated with the Green-Med diet, to improved glycemic control and slower brain aging. While further research is needed to fully understand the mechanisms at play, these results suggest a potential avenue for reducing the risk of age-related cognitive decline through relatively simple dietary adjustments.
The DIRECT PLUS trial was funded by grants from the German Research Foundation (DFG), Israel Ministry of Health, Israel Ministry of Science and Technology, and the California Walnuts Commission. None of the funding providers were involved in any stage of the design, conduct, or analysis of the study, nor did they have access to the study results before publication.
Reference:
Dafna Pachter, Alon Kaplan, Gal Tsaban, Hila Zelicha, Anat Yaskolka Meir, Ehud Rinott, Gidon Levakov, Moti Salti, Yoram Yovell, Sebastian Huhn, Frauke Beyer, Veronica Witte, Peter Kovacs, Martin von Bergen, Uta Ceglarek, Matthias Blüher, Michael Stumvoll, Frank B Hu, Meir J Stampfer, Alon Friedman, Ilan Shelef, Galia Avidan, Iris Shai, Glycemic control contributes to the neuroprotective effects of Mediterranean and green-Mediterranean diets on brain age: the DIRECT PLUS brain-magnetic resonance imaging randomized controlled trial, The American Journal of Clinical Nutrition, 2024, https://doi.org/10.1016/j.ajcnut.2024.09.013.
Powered by WPeMatico
