Brain molecule reverses movement deficits of Parkinson’s, offering new therapeutic target
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

Bengaluru: Biocon Biologics Limited, a subsidiary of Biocon Limited, has announced the successful pricing of Biocon Biologics’ debut USD 800 million (INR 66,763 million) senior secured Notes due 2029 at a coupon of 6.67%. The Bonds will be issued by Biocon Biologics Global plc which is a wholly owned subsidiary of BBL and will be backed by a strong security package. The Bonds are expected to be rated BB by both S&P and Fitch and will be listed on the Singapore Stock Exchange. The transaction is expected to settle on October 09, 2024 subject to customary closing conditions.
Powered by WPeMatico
New Delhi: In a move towards empowerment and inclusivity, centre-run Safdarjung Hospital has launched a unique initiative that highlights clothing and other items created by patients with disabilities. This initiative aims to provide a platform for these individuals to express their creativity while working towards financial independence.
On Friday, the hospital inaugurated a four-day sale featuring a diverse range of items, including clothing, blanket covers, and dolls, all crafted by patients. Having been paused during the pandemic, this initiative has now resumed, underscoring the hospital’s commitment to skill development and the empowerment of its patients.
Organized by the Vocational Guidance (VG) section of the Physical Medicine and Rehabilitation Department, the sale aims to upskill Persons with Disabilities (PwDs) and provide them with employment opportunities based on their newfound skills. The VG section has been actively offering training sessions to doctors, teachers, and craft instructors, benefiting both in-patients and out-patients.
According to The Hindu, Dr Suman Badhal, the faculty in charge of the VG Section and Staff, stated, “The hospital undertakes this initiative in an attempt to skill people who have disabilities so that they can get employment based on skills such as stitching.” She added that around 50-60 patients contributed to the items that were up for sale. Most of them, she added, have locomotor disabilities and some have spinal injuries.
The initiative goes beyond simply selling handcrafted items; it signifies a crucial step toward rehabilitation and empowerment. Dr Vandana Talwar, the medical superintendent of the hospital, highlighted that as a healthcare institution, there is a responsibility to extend beyond medical care and contribute to the holistic development of individuals. She emphasized that this sale exemplifies how rehabilitation can profoundly transform lives, showcasing the potential for creativity and independence among patients.
Through initiatives like this, Safdarjung Hospital is setting a precedent for how healthcare institutions can enhance the overall well-being and empowerment of individuals. By equipping patients with the skills to create marketable goods, the hospital actively contributes to transforming lives, providing patients with opportunities to integrate into society and experience a sense of accomplishment. This approach not only fosters creativity but also promotes independence, reinforcing the hospital’s commitment to holistic care.
Also Read: Delhi: Centre to increase budget of equipment aids for disabled persons
Powered by WPeMatico

New Delhi: Delhi Health Minister Saurabh Bharadwaj has announced the extension of tenure for current senior residents (SRs) and junior residents (JRs) in all government hospitals while new recruitments are underway.
The Department of Health and Family Welfare has also authorised all government hospitals to recruit JRs and SRs on an ad-hoc basis, following existing guidelines and orders until further notice, according to an order issued on Thursday, news agency PTI reported.
Meanwhile, the tenure of existing ad-hoc SRs and JRs in all government hospitals will also be extended until the recruitment process by the Centralised Committee is finalised, the order reads.
Also Read:JIPMER Faculty Association continue protests against Director’s Tenure extension
Delhi Health Minister Saurabh Bharadwaj announced the decision by posting the order on social media.
According to the PTI report, earlier this week, the health minister directed the health secretary to extend the tenure of junior and senior resident doctors in Delhi government hospitals for three months to prevent any disruption.
“Concerns were raised about the discontinuation of junior and senior resident doctors in Delhi government hospitals while new ones join through the Centralised Committee. This would cause a disruption of essential health services,” Bharadwaj said earlier.
“Therefore, I have directed the secretary (health) to extend their tenure for three months or until the new staff joins,” he added.
Medical Dialogues team had earlier reported that addressing a key concern over the recruitment process of junior and senior resident doctors, the Delhi State Health Department recently approved the formation of a centralized committee to look after the recruitment of doctors and directed hospitals not to engage in direct recruitment. The committee will have four members including a resident doctor and will be led by the Dean of Maulana Azad Medical College (MAMC). Due to the creation of the committee, hospitals will no longer have the authority to directly recruit junior and senior resident doctors.
Also Read:Punjab Govt to reduce bond tenure for doctors, increase amount
Powered by WPeMatico
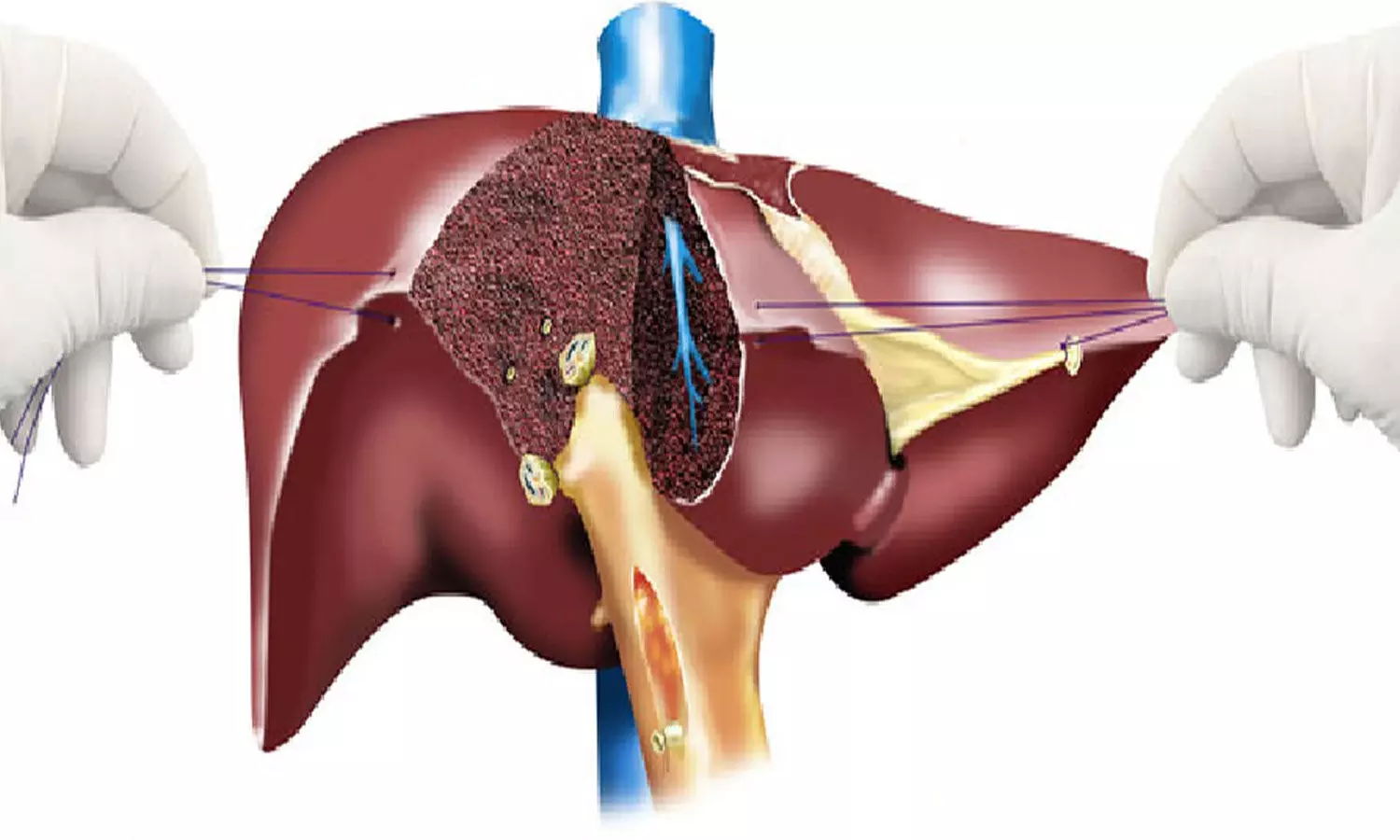
HEADING: Tailoring Pain Management: Study Sheds Light on Enhancing Living Liver Donor Outcomes Through Innovative Analgesia
India: A recent review published online in Apollo Medicine has shed light on the evolving strategies for pain management in living liver donors undergoing hepatectomy.
The review emphasizes the need for tailored analgesic approaches that consider individual patient characteristics and requirements. It highlights the potential advantages of adopting innovative methods to improve pain management, decrease opioid use, and ultimately enhance outcomes and satisfaction for living liver donors undergoing hepatectomy.
Speaking on the study’s relevance in the current medical landscape, the lead author, Dr. Ashish Malik, the Department of Anaesthesia and Critical Care at Indraprastha Apollo Hospital in New Delhi, India, shared his insights with Medical Dialogues:
“Donor analgesia has been of paramount importance for the development of the living donor liver transplant program, serving as the cornerstone for motivating donation. Multiple methods for providing pain relief have been adopted. This article contributes to discussing the merits and demerits of various techniques and explores how a multimodal approach can be formulated to ensure donor safety.

Dr. Malik also highlighted the unique aspects of the study, stating, “This review article provides in-depth knowledge about all available methods for donor analgesia, based on which a blended approach can be formulated.” His insights emphasize the comprehensive nature of the research, which aims to enhance pain management strategies for living liver donors.
The study authors note that donor hepatectomy is a complex surgical procedure that often leads to considerable postoperative pain, affecting both short-term recovery and long-term outcomes. Effective pain management is essential for the well-being of living liver donors and for optimizing their overall experience. The review by Dr. Malik and the team seeks to offer a thorough overview of current practices and emerging strategies in analgesic management for donor hepatectomy.
For this purpose, the team conducted searches on PubMed and Google Scholar to identify relevant materials. Their evaluation included review articles, clinical trials, retrospective studies, observational studies, and case-control studies. The review explores recent advancements in pain management, including enhanced recovery after surgery protocols, personalized analgesic regimens, and innovative pharmaceutical agents. Additionally, it examines the role of psychological factors and patient-centered care in shaping postoperative pain experiences.
The review revealed the following findings:
Dr. Malik highlighted the implications and key findings of the review, stating that the article aims to enhance recovery after surgery by establishing a standardized approach to pain management. He emphasized the significance of regional anesthesia techniques and the use of novel pharmaceutical agents such as magnesium, dexmedetomidine, gabapentin, and ketamine, all of which can help reduce opioid consumption during these procedures. As surgical methods evolve from open donor hepatectomies to laparoscopic and robotic techniques, these strategies are becoming increasingly relevant.
However, Dr. Malik noted that there is still limited research data in the field of donor analgesia. Randomized controlled trials involving large patient populations are necessary to determine the most effective techniques for managing donor pain. He stressed the importance of focusing future research on acute and chronic pain scores in living donors, as these factors directly influence the quality of life for donors.
Reference:
Malik, A., & Pal, A. (2024). Analgesia for Donor Hepatectomy: Recent Perspectives. Apollo Medicine.https://doi.org/10.1177/09760016241248310
Powered by WPeMatico

A recent study by Samuel Dicken and team suggests concentrating on cutting back on the consumption of particular ultra-processed foods (UPF) in order to minimize the risk of type 2 diabetes. The findings of this study were published in The Lancet Regional Health – Europe journal.
People who have type 2 diabetes mellitus also have a higher risk of all-cause mortality and other cardiometabolic disorders, which lowers their quality of life. The question of whether diets heavy in ultra-processed foods (UPF) present health concerns in addition to those related to diet quality is up for dispute. UPF consumption is rising globally and accounts for a considerable amount of dietary intake. Increased UPF consumption have been linked to detrimental health effects, including as obesity and weight gain, as well as cardiovascular disease. Thus, the relationship between the degree of food processing and the occurrence of type 2 diabetes mellitus was investigated in this study.
The European Prospective Investigation into Cancer and Nutrition (EPIC) prospective cohort analysis was used for this study. Using dietary questionnaires, dietary consumption was measured at baseline and categorized into four categories namely, processed food (PF), unprocessed/minimally processed food (MPF), processed culinary ingredients (PCI), and UPF. Cases of type 2 diabetes mellitus were confirmed using a variety of techniques. In order to assess the correlations between MPF + PCI, PF, and UPF consumption and incidence type 2 diabetes mellitus, Cox regression and statistical substitution analysis were employed. UPF sub-group analysis was done to look into any heterogeneity in the relationship between UPF and incidence type 2 diabetes mellitus.
Overall, higher UPF consumption was linked with an increase in incidence type 2 diabetes mellitus, while higher intakes of less processed foods were associated with a decrease in the incidence of type 2 diabetes mellitus.
Source:
Dicken, S. J., Dahm, C. C., Ibsen, D. B., Olsen, A., Tjønneland, A., Louati-Hajji, M., Cadeau, C., Marques, C., Schulze, M. B., Jannasch, F., Baldassari, I., Manfredi, L., Santucci de Magistris, M., Sánchez, M.-J., Castro-Espin, C., Palacios, D. R., Amiano, P., Guevara, M., van der Schouw, Y. T., … Batterham, R. L. (2024). Food consumption by degree of food processing and risk of type 2 diabetes mellitus: a prospective cohort analysis of the European Prospective Investigation into Cancer and Nutrition (EPIC). In The Lancet Regional Health – Europe (p. 101043). Elsevier BV. https://doi.org/10.1016/j.lanepe.2024.101043
Powered by WPeMatico

Novo Nordisk today announced that in a phase 2a trial, monlunabant, a novel oral cannabinoid receptor 1 inverse agonist, was associated with weight loss in people with obesity and metabolic syndrome. Monlunabant, formerly INV-202, was part of the acquisition of Inversago Pharmaceuticals Inc. announced in August 2023.
The trial investigated the efficacy and safety of a once-daily 10 mg, 20 mg and 50 mg dose of monlunabant compared to placebo on body weight after 16 weeks in 243 people with obesity and metabolic syndrome1. People were equally randomised among the four treatment arms.
From a baseline body weight of 110.1 kg, all doses of monlunabant achieved a statistically significant weight loss compared to placebo. After 16 weeks of treatment, people treated with a once-daily 10 mg dose of monlunabant achieved a weight loss of 7.1 kg compared to a reduction of 0.7 kg with placebo2. Limited additional weight loss was seen at higher doses of monlunabant.
In the trial, the most common adverse events were gastrointestinal, with the vast majority being mild to moderate and dose dependent. Reporting of mild to moderate neuropsychiatric side effects, primarily anxiety, irritability, and sleep disturbances, was more frequent and dose dependent with monlunabant compared to placebo. No serious adverse events were reported in relation to neuropsychiatric side effects.
“The phase 2a results indicate the weight-lowering potential of monlunabant and that further work is needed to determine the optimal dosing to balance safety and efficacy,” said Martin Holst Lange, executive vice president and head of Development at Novo Nordisk. “Obesity is a complex disease with a significant unmet need, and as an oral small molecule having a new mechanism of action, monlunabant is one of the novel projects in our pipeline with the potential of treating obesity.”
Based on the results, Novo Nordisk expects to initiate a larger phase 2b trial in obesity to further investigate dosing and the safety profile of monlunabant over a longer duration in a global population. The phase 2b trial is expected to be initiated in 2025.About monlunabant and CB1 Monlunabant is an inverse agonist of the CB1 receptor which plays an important role in metabolism and appetite regulation in the central nervous system as well as in peripheral tissues such as adipose tissues, the gastrointestinal tract, kidneys, liver, pancreas, muscles and lungs. CB1 plays an important role in appetite regulation and cardiometabolic pathways.
Monlunabant is an inverse agonist of the CB1 receptor which plays an important role in metabolism and appetite regulation in the central nervous system as well as in peripheral tissues such as adipose tissues, the gastrointestinal tract, kidneys, liver, pancreas, muscles and lungs. CB1 plays an important role in appetite regulation and cardiometabolic pathways.
Powered by WPeMatico
